Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Strength | 40 mg |
| Treatment | Breast Cancer |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
Dosage
As per Doctor's Prescription
Storage
Cool and Dry Place
Best Before
36 Months from Manufacturing
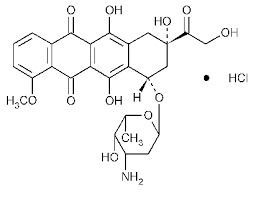
FDA approves neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer. On July 17, 2017, the U.S. Food and Drug Administration approved neratinib (NERLYNX, Puma Biotechnology, Inc.) for the extended adjuvant treatment of adult patients with early stage HER2- verexpressed / amplified breast cancer, to follow adjuvant trastuzumab-based therapy.
NERLYNX is a kinase inhibitor indicated for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy.
Looking for "Neratinib Tablets" ?
Explore More Products