Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
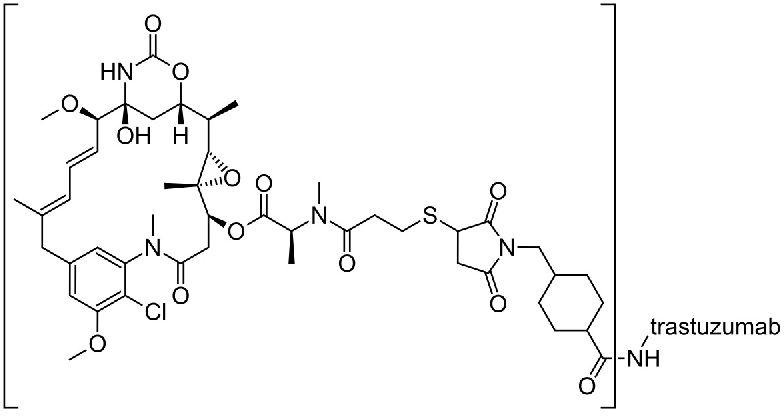
Breast Cancer
Leading Exporters and Wholesaler of Ado-Trastuzumab Emtansine for Injection, Anastrozole Tablets, Eribulin Mesylate Injection, Fulvestrant Injection, Lapatinib Tablets, Letrozole Tablets, Neratinib Tablets, Paclitaxel Injection, Ribociclib and Letrozole Tablets, Trastuzumab Intravenous Infusion and Vindesine Sulphate Powder for Injection from Delhi.
| Business Type | Exporter, Supplier |
| Usage/Application | Medicine |
| Usage | Used to Treat Advanced TNBC |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| Treatment | Advanced Breast Cancer |
| Form | Tablets |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
The FDA approved the use of anastrozole (Arimidex) for the adjuvant treatment of hormone-receptor positive early breast cancer in postmenopausal women.
In September 2002, the FDA approved a supplemental new drug application (sNDA) for Arimidex under the provisions for accelerated approval, with further follow-up required for full approval. The supplemental approval was based on recurrence-free survival data from the Arimidex Tamoxifen Alone or in Combination (ATAC) trial with a median duration treatment of 31 months.
ARIMIDEX is an aromatase inhibitor indicated for:
- Adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer
- First-line treatment of postmenopausal women with hormone receptor-positive or hormone receptor unknown locally advanced or metastatic breast cancer
- Treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. Patients with ER-negative disease and patients who did not respond to previous tamoxifen therapy rarely responded to ARIMIDEX
| Business Type | Exporter, Supplier |
| API Form | Liquid |
| Dosage | As per Doctor's Prescription |
| Type | Intravenous Administration |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
On January 28, 2016, the U. S. Food and Drug Administration approved eribulin (HALAVEN injection, Eisai Co., Ltd.) for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
HALAVEN is a microtubule inhibitor indicated for the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting .
| Business Type | Exporter, Supplier |
| Shelf Life | 1 years |
| API Form | Liquid |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Treatment | To Treat a Certain Type of Advanced Breast Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Dosage | As per Doctor's Prescription |
| Treatment | Cancer |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
Are you looking for or in search of FDA approved “Letrozole tablets in India”? “Medicant Healthcare” a registered pharmacy firm in India deals in both brand name as well as generic name Drugs / medicine approved for Breast cancer treatment. Need Access of “Letrozole tablets, get in touch with our healthcare professional.
Femara is an aromatase inhibitor indicated for:
- Adjuva nt treatment of postmenopausal women with hormone receptor positive early brea st cancer
- Extended adjuvant treatment of postmenopausal women with early brea st cancer who have received prior standard adju vant ta moxifen thera py
- First a nd second-line treatment of postmenopausal women with hormone receptor positive or unknown advanced breast cancer
DOSAGE AND ADMINISTRATION
- Femara tablets are taken orally without regard to meals
- Recommended dose: 2.5.mg once daily
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Strength | 40 mg |
| Treatment | Breast Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA approves neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer. On July 17, 2017, the U.S. Food and Drug Administration approved neratinib (NERLYNX, Puma Biotechnology, Inc.) for the extended adjuvant treatment of adult patients with early stage HER2- verexpressed / amplified breast cancer, to follow adjuvant trastuzumab-based therapy.
NERLYNX is a kinase inhibitor indicated for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy.
| Business Type | Exporter, Supplier |
| Treatment | Breast Cancer |
| Dosage Form | Injection |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug used alone or with other drugs to treat AIDS-related Kaposi sarcoma, advanced ovarian cancer, and certain types of breast cancer and non-small cell lung cancer. It is also being studied in the treatment of other types of cancer. Paclitaxel stops cancer cells from growing and dividing and may kill them. It is a type of taxane.
Paclitaxel (PTX), sold under the brand name Taxol among others, is a chemotherapy medication used to treat a number of types of cancer. This includes ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi’s sarcoma, cervical cancer, and pancreatic cancer. It is administered by intravenous injection. There is also an albumin-bound formulation.
Paclitaxel is approved to be used alone or with other drugs to treat:
AIDS-related Kaposi sarcoma. It is used as second-line therapy.
Breast cancer. It is used:
- Non-small cell lung cancer. It is used with cisplatin as first-line therapy in patients whose disease cannot be treated with surgery or radiation therapy.
- Ovarian cancer that is advanced. It is used with cisplatin as first-line therapy or alone in patients who have already received other treatment.
| Business Type | Exporter, Supplier |
| Brand Name(s) | Kisqali Femara Co-Pack |
| Generic Name | Ribociclib and Letrozole |
| Dosage Form(s) | Tablets |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. On July 18, 2018, the Food and Drug Administration expanded the indication for ribociclib (Kisqali, Novartis Pharmaceuticals Corporation) in combination with an aromatase inhibitor for pre/perimenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer, as initial endocrine-based therapy.
The Kisqali Femara Co-Pack is a prescription that contains two medications, ribociclib (Kisqali) and letrozole (Femara), used as a first-line treatment for adults who have advanced or metastatic hormone-receptor-positive human epidermal growth factor-2 receptors (HER2)-negative breast cancer.1 Metastatic cancer is cancer that has spread from the primary site to other areas of the body.
- Generic Name: Ribociclib and letrozole
- Brand Name(s): Kisqali Femara Co-Pack
- Drug Availability: Prescription
- Administration Route: Oral
- Therapeutic Classification: CDK4/6 inhibitor and aromatase inhibitor
- Available Generically: No
- Active Ingredient: Ribociclib and letrozole
- Dosage Form(s): Tablet
| Business Type | Exporter, Supplier |
| Application | Clinic, Hospital |
| Usage | Used for Cancer Treatment |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| Form | Powder |
| Treatment | For Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
Vindesine, also termed Eldisine, is a semisynthetic vinca alkaloid derived from the flowering plant Catharanthus roseus. Like the natural (e.g. vinblastine and vincristine) and semisynthetic vinca alkaloids (e.g. vinorelbine and vinflunine) derived from this plant, vindesine is an inhibitor of mitosis that is used as a chemotherapy drug. By inhibiting mitosis, vinedsine blocks the proliferation of cells, particularly the rapidly proliferation cells of certain types of cancer. It is used, generally in combination with other chemotherapeutic drugs, in the treatment of various malignancies such as leukaemia, lymphoma, melanoma, breast cancer, and lung cancer