Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
| Business Type | Exporter, Supplier |
| Treatment | Cancer |
| Form | Tablets |
| Dosage | As per Doctor's Prescription |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
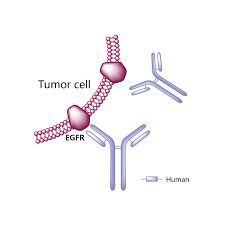
A drug used alone to treat certain types of non-small cell lung cancer and with gemcitabine hydrochloride to treat certain types of pancreatic cancer. It is also being studied in the treatment of other types of cancer. Erlotinib hydrochloride blocks a protein called EGFR, which may help keep cancer cells from growing. It is a type of tyrosine kinase inhibitor. Also called CP-358,774, OSI-774, and Tarceva. US Brand Name(s): Tarceva. FDA Approved: Yes
Erlotinib, sold under the brand name Tarceva among others, is a medication used to treat non-small cell lung cancer (NSCLC) and pancreatic cancer. Specifically it is used for NSCLC with mutations in the epidermal growth factor receptor (EGFR) — either an exon 19 deletion (del19) or exon 21 (L858R) substitution mutation — which has spread to other parts of the body. It is taken by mouth.
Erlotinib was approved for medical use in the United States in 2004. It is on the World Health Organization’s List of Essential Medicines.
Use in Cancer
- Erlotinib hydrochloride is approved to be used alone or with other drugs to treat:
- Non-small cell lung cancer (NSCLC) that is metastatic and has certain EGFR gene mutations.
- The use of erlotinib hydrochloride to treat NSCLC that does not have the EGFR gene mutations is no longer FDA-approved.
- Pancreatic cancer. It is used with gemcitabine hydrochloride in patients whose disease cannot be removed by surgery, is locally advanced, or has metastasized.
- Erlotinib hydrochloride is also being studied in the treatment of other types of cancer.
Looking for "Erlotinib Tablets" ?
Explore More Products