Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
Pancreatic Cancer
Leading Exporters and Wholesaler of Erlotinib Tablets, gemcitabine injection and Sunitinib Malate Capsules from Delhi.
| Business Type | Exporter, Supplier |
| Treatment | Cancer |
| Form | Tablets |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
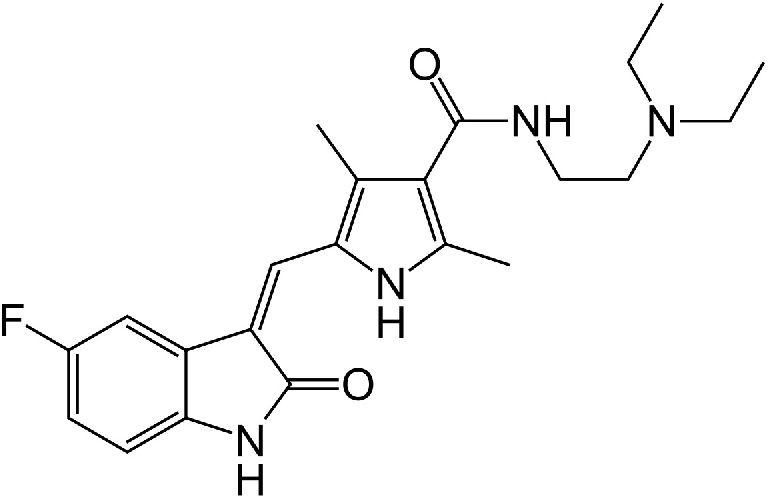
A drug used alone to treat certain types of non-small cell lung cancer and with gemcitabine hydrochloride to treat certain types of pancreatic cancer. It is also being studied in the treatment of other types of cancer. Erlotinib hydrochloride blocks a protein called EGFR, which may help keep cancer cells from growing. It is a type of tyrosine kinase inhibitor. Also called CP-358,774, OSI-774, and Tarceva. US Brand Name(s): Tarceva. FDA Approved: Yes
Erlotinib, sold under the brand name Tarceva among others, is a medication used to treat non-small cell lung cancer (NSCLC) and pancreatic cancer. Specifically it is used for NSCLC with mutations in the epidermal growth factor receptor (EGFR) — either an exon 19 deletion (del19) or exon 21 (L858R) substitution mutation — which has spread to other parts of the body. It is taken by mouth.
Erlotinib was approved for medical use in the United States in 2004. It is on the World Health Organization’s List of Essential Medicines.
Use in Cancer
- Erlotinib hydrochloride is approved to be used alone or with other drugs to treat:
- Non-small cell lung cancer (NSCLC) that is metastatic and has certain EGFR gene mutations.
- The use of erlotinib hydrochloride to treat NSCLC that does not have the EGFR gene mutations is no longer FDA-approved.
- Pancreatic cancer. It is used with gemcitabine hydrochloride in patients whose disease cannot be removed by surgery, is locally advanced, or has metastasized.
- Erlotinib hydrochloride is also being studied in the treatment of other types of cancer.
| Business Type | Exporter, Supplier |
| API Form | Powder |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| Form | Capsules |
| Treatment | Used to Treat Adults with Certain Types of Gastrointestinal Stromal Tumors, |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
On November 16, 2017, the Food and Drug Administration approved sunitinib malate for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma following nephrectomy.On November 16, 2017, the Food and Drug Administration approved sunitinib malate (Sutent, Pfizer Inc.) for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma following nephrectomy.Sunitinib malate capsules belongs to a class of drugs called Antineoplastics, Tyrosine Kinase Inhibitor; Antineoplastics, VEGF Inhibitor.
A drug used to treat adults with certain types of gastrointestinal stromal tumors, pancreatic neuroendocrine tumors, or renal cell carcinoma (a type of kidney cancer). It is also being studied in the treatment of other types of cancer. Sunitinib malate blocks certain proteins, which may help keep cancer cells from growing. It may also prevent the growth of new blood vessels that tumors need to grow. Sunitinib malate is a type of tyrosine kinase inhibitor and a type of antiangiogenesis agent. Sunitinib is the active ingredient of sunitinib malate. Also called SU011248, SU11248, and Sutent. US Brand Name(s): Sutent. FDA Approved: Yes
Sunitinib, sold under the brand name Sutent, is a medication used to treat cancer. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) on January 26, 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.
Sunitinib malate is approved to treat adults with:
- Gastrointestinal stromal tumor (a type of stomach cancer). It is used in patients whose condition has become worse while taking imatinib mesylate or who are not able to take it.
- Pancreatic cancer. It is used in patients with progressive neuroendocrine tumors that cannot be removed by surgery, are locally advanced, or have metastasized (spread to other parts of the body).
- Renal cell carcinoma (a type of kidney cancer).
- Sunitinib malate is also being studied in the treatment of other types of cancer.