Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
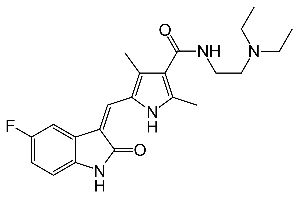
FDA Approves Capecitabine, First Oral Chemotherapy for the Treatment of Metastatic Colorectal Cancer. The US Food and Drug Administration (FDA) has approved capecitabine (Xeloda), the first oral chemotherapy for the treatment of metastatic colorectal cancer (the second leading cause of cancer mortality among Americans).
The FDA has approved the first generic formulation of capecitabine (Xeloda), an oral chemotherapeutic that is currently approved to treat patients with metastatic colorectal cancer (mCRC) and metastatic breast cancer (MBC). XELODA (capecitabine) is a nucleoside metabolic inhibitor with antineoplastic activity indicated for:
- Adjuv ant Colon Cancer
- Metastatic Colorectal Cancer
- Metastatic Breast Cancer
Looking for "Capecitabine Tablets" ?
Explore More Products