Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
Colorectal Cancer
Leading Exporters and Wholesaler of Bevacizumab Concentrate for Solution for Infusion, Capecitabine Tablets, Irinotecan Injection and Panitumumab Solution for Intravenous Infusion from Delhi.
| Business Type | Exporter, Supplier |
| Form | Liquid |
| Treatment | Cervical Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug that binds to the protein VEGF to help keep new blood vessels from forming and is used to treat many different types of cancer. Bevacizumab is used under the brand names Alymsys, Mvasi, Avastin, and Zirabev, alone or with other drugs, to treat certain types of cervical cancer, colorectal cancer, non-small cell lung cancer, renal cell carcinoma (a type of kidney cancer), and glioblastoma (a type of brain cancer). The Alymsys and Avastin brands of bevacizumab are also used to treat certain types of ovarian epithelial, fallopian tube, and primary peritoneal cancer. The Avastin brand is also used to treat certain types of hepatocellular carcinoma (a type of liver cancer). Bevacizumab is also being studied in the treatment of other types of cancer. It may prevent the growth of new blood vessels that tumors need to grow. Bevacizumab is a type of antiangiogenesis agent and a type of monoclonal antibody. US Brand Name(s): Alymsys, Avastin, Mvasi, Zirabev. FDA Approved: Yes
Bevacizumab, sold under the brand name Avastin among others, is a medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, glioblastoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).
Bevacizumab is approved to be used alone or with other drugs to treat:
Cervical cancer that has not gotten better with other treatment, has metastasized (spread to other parts of the body), or has recurred (come back). It is used with paclitaxel and either cisplatin or topotecan hydrochloride. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Colorectal cancer that has metastasized. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Hepatocellular carcinoma (a type of liver cancer) that is metastatic or cannot be removed by surgery. It is used with atezolizumab in patients who have not received systemic therapy. This use is approved for the Avastin brand of bevacizumab.
Nonsquamous non-small cell lung cancer that is locally advanced, cannot be removed by surgery, has metastasized, or has recurred. It is used with carboplatin and paclitaxel as first-line therapy. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Ovarian epithelial, fallopian tube, or primary peritoneal cancer.
Renal cell carcinoma (a type of kidney cancer) that has metastasized. It is used with interferon alpha. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Bevacizumab is also being studied in the treatment of other types of cancer.
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA Approves Capecitabine, First Oral Chemotherapy for the Treatment of Metastatic Colorectal Cancer. The US Food and Drug Administration (FDA) has approved capecitabine (Xeloda), the first oral chemotherapy for the treatment of metastatic colorectal cancer (the second leading cause of cancer mortality among Americans).
The FDA has approved the first generic formulation of capecitabine (Xeloda), an oral chemotherapeutic that is currently approved to treat patients with metastatic colorectal cancer (mCRC) and metastatic breast cancer (MBC). XELODA (capecitabine) is a nucleoside metabolic inhibitor with antineoplastic activity indicated for:
- Adjuv ant Colon Cancer
- Metastatic Colorectal Cancer
- Metastatic Breast Cancer
| Business Type | Exporter, Supplier |
| Form | Liquid |
| Packaging Type | Glass Bottles |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug used alone or with other drugs to treat colorectal cancer that has spread to other parts of the body. It is used in patients whose cancer has not already been treated or whose cancer has recurred (come back) or has gotten worse after treatment with anticancer drugs that included fluorouracil. It is also being studied in the treatment of other types of cancer. Camptosar blocks a certain enzyme needed for cell division and DNA repair, and it may kill cancer cells. It is a type of topoisomerase inhibitor and a type of camptothecin analog. Also called CPT 11 and irinotecan hydrochloride.
Irinotecan, sold under the brand name Camptosar among others, is a medication used to treat colon cancer, and small cell lung cancer.[4] For colon cancer it is used either alone or with fluorouracil. For small cell lung cancer it is used with cisplatin. It is given intravenously. Irinotecan was approved for medical use in the United States in 1996. It is on the World Health Organization’s List of Essential Medicines.[6] It is made from the natural compound camptothecin which is found in the Chinese ornamental tree Camptotheca acuminata.
Its main use is in colon cancer, in particular, in combination with other chemotherapy agents. This includes the regimen FOLFIRI, which consists of infusional 5-fluorouracil, leucovorin, and irinotecan. The regimen XELIRI consists of capecitabine and irinotecan.
It may also be used together with fluorouracil and folinic acid for pancreatic cancer following failure of initial treatment.
| Business Type | Exporter, Supplier |
| Form | Liquid |
| Usage | Colorectal |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
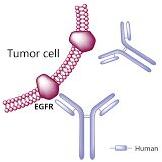
panitumumab Solution for Intravenous Infusion
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.