Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
Prostate Cancer
Leading Exporters and Wholesaler of Abiraterone Acetate Tablets, Apalutamide Tablets, Docetaxel Injection, Enzalutamide Capsules and Goserelin Acetate Implant Injection from Delhi.
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Treatment | Used to Treat Prostate Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug used with other drugs to treat prostate cancer that has spread to other parts of the body. Abiraterone acetate is used under the brand name Zytiga to treat patients whose cancer is castration resistant (has not responded to treatments that lower testosterone levels) or whose cancer is high risk and castration sensitive (has responded to treatments that lower testosterone levels). It is also used under the brand name Yonsa to treat patients whose cancer is castration resistant. Abiraterone acetate is also being studied in the treatment of other types of cancer. It lowers the amount of androgens (male hormones), such as testosterone, made by the body. This may stop the growth of cancer cells that need androgens to grow. Abiraterone acetate is a type of antiandrogen. US Brand Name(s): Yonsa Zytiga. FDA Approved: Yes
Abiraterone acetate, sold under the brand name Zytiga among others, is a medication used to treat prostate cancer. Specifically it is used together with a corticosteroid for metastatic castration-resistant prostate cancer (mCRPC) and metastatic high-risk castration-sensitive prostate cancer (mCSPC). It should either be used following removal of the testicles or along with a gonadotropin-releasing hormone (GnRH) analog. It is taken by mouth.
Abiraterone acetate is approved to be used with prednisone to treat:
- Prostate cancer that has metastasized (spread to other parts of the body). It is used:
- In patients whose cancer is castrate resistant (has not responded to treatments that lower testosterone levels). This use is approved for the Zytiga brand name of abiraterone acetate.
- With methylprednisolone in patients whose cancer is castrate resistant. This use is approved for the Yonsa brand name of abiraterone acetate.
- In patients whose cancer is high-risk and castrate sensitive (has responded to treatments that lower testosterone levels). This use is approved for the Zytiga brand name of abiraterone acetate.
- Abiraterone is also being studied in the treatment of other types of cancer.
| Business Type | Exporter, Supplier |
| Treatment | Cancer |
| Form | Tablets |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| Form | Liquid |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug used alone or with other drugs to treat certain types of breast cancer, stomach cancer, gastroesophageal junction cancer, non-small cell lung cancer, prostate cancer, and squamous cell carcinoma of the head and neck. It is also being studied in the treatment of other types of cancer. Docetaxel stops cancer cells from growing and dividing and may kill them. It is a type of taxane. Also called Taxotere. US Brand Name(s): Taxotere. FDA Approved: Yes
Docetaxel is approved to be used alone or with other drugs to treat:
- Breast cancer that is locally advanced or has metastasized (spread to other parts of the body) and has not gotten better with other chemotherapy. It is also used with doxorubicin hydrochloride and cyclophosphamide to treat breast cancer that is node-positive and can be removed by surgery.
- Non-small cell lung cancer that is locally advanced or has metastasized. It is used:
- Alone in patients whose cancer has not gotten better after platinum chemotherapy; or
- With cisplatin in patients whose cancer cannot be treated with surgery.
- Prostate cancer that has metastasized in men whose cancer is hormone-refractory (does not respond to hormone treatment).
- Squamous cell carcinoma of the head and neck that is locally advanced. It is used with cisplatin and fluorouracil.
- Stomach adenocarcinoma or gastroesophageal junction adenocarcinoma (a rare type of esophageal cancer) that is advanced. It is used in patients whose disease has not been treated with chemotherapy.
| Business Type | Exporter, Supplier |
| Dosage Form | Capsules |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug used to treat prostate cancer that has spread to other parts of the body and is castration sensitive (has responded to treatments that lower testosterone levels) and prostate cancer that is castration resistant (has not responded to treatments that lower testosterone levels). It is also being studied in the treatment of other types of cancer. Enzalutamide binds to proteins called androgen receptors, which are found in some prostate cancer cells. These proteins bind to androgens (male hormones) and may cause cancer cells to grow. Enzalutamide blocks these proteins and may keep cancer cells from growing. It is a type of antiandrogen. Also called Xtandi. US Brand Name(s): Xtandi. FDA Approved Yes.
Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
Enzalutamide was first described in 2006, and was introduced for the treatment of prostate cancer in 2012. It was the first second-generation NSAA to be introduced. It is on the World Health Organization’s List of Essential Medicines.
Enzalutamide is approved to treat: Prostate cancer. Enzalutamide is also being studied in the treatment of other types of cancer.
| Business Type | Exporter, Supplier |
| Treatment | Cancer |
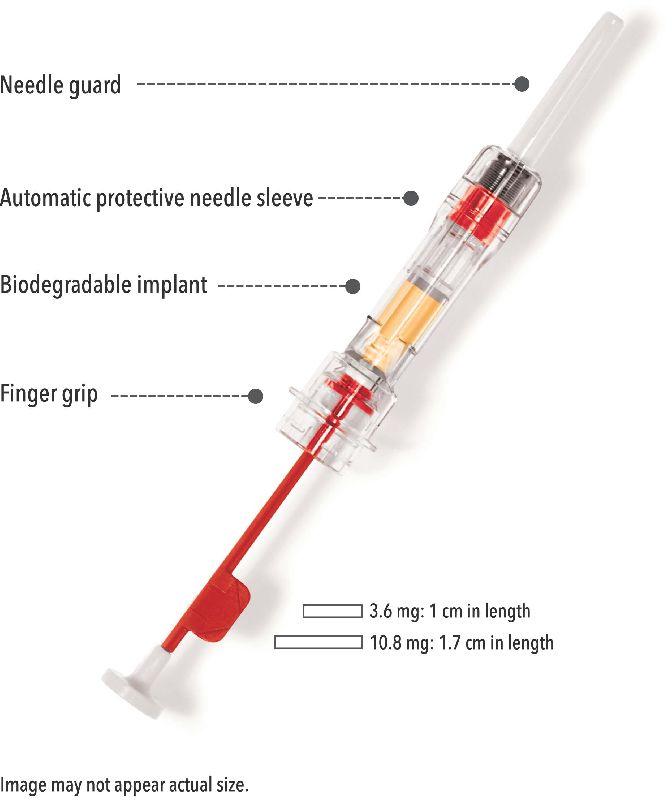
| Dosage form | Injection |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.