Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
Lung Cancer
Leading Exporters and Wholesaler of Afatinib Tablets, Atezolizumab Injection, Ceritinib Hard Gelatin Capsules, Durvalumab Injection, Macitentan Tablets, Osimertinib Tablets, Rivaroxaban for Oral Suspension, Selexipag Tablets and Vinorelbine Injection from Delhi.
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Treatment | Cancer Treatment |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| API Form | Liquid |
| Usage | Treatment of Urothelial Carcinoma, Non-small Cell Lung Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug that binds to the protein PD-L1 to help immune cells kill cancer cells better and is used to treat many different types of cancer, including cancers that express PD-L1. Atezolizumab is used alone or with other drugs to treat certain types of melanoma, hepatocellular carcinoma (a type of liver cancer), non-small cell lung cancer, small cell lung cancer, and urothelial cancer (a type of cancer in the bladder or urinary tract). It is also being studied in the treatment of other types of cancer. Atezolizumab may block PD-L1 and help the immune system kill cancer cells. It is a type of monoclonal antibody and a type of immune checkpoint inhibitor. Also called Tecentriq. US Brand Name(s): Tecentriq. FDA Approved: Yes
In the European Union atezolizumab is indicated for the treatment of urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma, and breast cancer.
In the United States, atezolizumab is indicated for the treatment of urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), hepatocellular carcinoma (HCC), and melanoma.
Atezolizumab is approved to treat:
- Hepatocellular carcinoma (a type of liver cancer) that is metastatic or cannot be removed by surgery. Atezolizumab is used with bevacizumab in patients who have not received systemic therapy.
- Melanoma that has a certain mutation in the BRAF gene. Atezolizumab is used with cobimetinib fumarate and vemurafenib in adults whose cancer is metastatic or cannot be removed by surgery.
- Non-small cell lung cancer.
- Small cell lung cancer. Atezolizumab is used with carboplatin and etoposide as the first treatment in adults with extensive-stage cancer.
- Urothelial cancer (a type of cancer in the bladder or urinary tract) that is metastatic or cannot be removed by surgery.
This use is approved under FDA’s Accelerated Approval Program. As a condition of approval, a confirmatory trial(s) must show that atezolizumab provides a clinical benefit in these patients.
Atezolizumab is also being studied in the treatment of other types of cancer.
| Business Type | Exporter, Supplier |
| Form | Hard Gelatin Capsules |
| Treatment | Cancer Treatment |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug used to treat adults with non-small cell lung cancer that has spread and is ALK positive. It is also being studied in the treatment of other types of cancer. Ceritinib blocks certain proteins made by the ALK gene. Blocking these proteins may stop the growth and spread of cancer cells. Ceritinib is a type of tyrosine kinase inhibitor. Also called Zykadia. US Brand Name(s): Zykadia. FDA Approved: Yes
Ceritinib is a prescription-only drug used for the treatment of non-small cell lung cancer (NSCLC). It was developed by Novartis and received FDA approval for use in April 2014.
Ceritinib is an anaplastic lymphoma kinase (ALK) inhibitor primarily used for the treatment of ALK positive metastatic NSCLC.[4][5] Previously, it was only indicated for patients who had developed resistant to crizotinib, another ALK inhibitor, but has since had its usage expanded to serve as a primary option for metastatic NSCLC.
Ceritinib is approved to treat: Non-small cell lung cancer that is ALK positive and has metastasized (spread to other parts of the body). It is used in adults.
| Business Type | Exporter, Supplier |
| API Form | Liquid |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug that binds to the protein PD-L1 to help immune cells kill cancer cells better and is used to treat different types of cancer. Durvalumab is used alone or with other drugs to treat adults with certain types of extensive-stage small cell lung cancer or stage III non-small cell lung cancer. It is also being studied in the treatment of other types of cancer. Durvalumab may block PD-L1 and help the immune system kill cancer cells. It is a type of monoclonal antibody and a type of immune checkpoint inhibitor. Also called Imfinzi. US Brand Name(s): Imfinzi. FDA Approved: Yes
Durvalumab is approved to treat:
- Non-small cell lung cancer (NSCLC). It is used in adults with stage III NSCLC that cannot be removed by surgery and did not get worse after platinum chemotherapy and radiation therapy.
- Small cell lung cancer in adults. It is used with etoposide phosphate and either carboplatin or cisplatin as the first treatment in adults with extensive-stage cancer.
- Durvalumab is also being studied in the treatment of other types of cancer.
The US Food and Drug Administration (FDA) has approved durvalumab for certain types of bladder and lung cancer:
- Adults with locally advanced or metastatic urothelial carcinoma who either have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
- Adults with unresectable, Stage III non-small cell lung cancer whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
- In combination with etoposide and either carboplatin or cisplatin, as first-line treatment for adults with extensive-stage small cell lung cancer.
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Brand Name | Opsumit |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
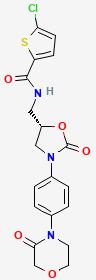
Macitentan is used to treat high blood pressure in the lungs (pulmonary arterial hypertension). This condition is thought to be caused by increased levels of a certain natural substance (endothelin-1). This medication blocks the effects of endothelin-1, which helps decrease the blood pressure in the lungs, and reduces the risk of worsening of symptoms and hospital stays due to the disease.
Macitentan, sold under the brand name Opsumit, is an endothelin receptor antagonist (ERA) developed by Actelion and approved for the treatment of pulmonary arterial hypertension (PAH). The other two ERAs marketed as of 2014 are bosentan and ambrisentan.[2] Macitentan is a dual ERA, meaning that it acts as an antagonist of two endothelin (ET) receptor subtypes, ETA and ETB.[2] However, macitentan has a 50-fold increased selectivity for the ETA subtype compared to the ETB subtype. The drug received approval from the U.S. Food and Drug Administration (FDA) on October 13, 2013.
| Business Type | Exporter, Supplier |
| Treatment | Cancer Treatment |
| Form | Tablets |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
A drug used to treat adults with some types of non-small cell lung cancer that have certain mutations (changes) in the EGFR gene. It is also being studied in the treatment of other types of cancer. Osimertinib mesylate blocks certain proteins made by the mutated EGFR gene, which may help keep cancer cells from growing and may kill them. It is a type of tyrosine kinase inhibitor. Also called Tagrisso. US Brand Name(s): Tagrisso. FDA Approved Yes
Osimertinib, sold under the brand name Tagrisso, is a medication used to treat non-small-cell lung carcinomas with specific mutations. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor. Osimertinib was approved for medical use in the United States in November 2015, and in the European Union in February 2016.
Osimertinib mesylate is approved to treat: Non-small cell lung cancer in adults whose tumors have certain EGFR gene mutations. Osimertinib mesylate is also being studied in the treatment of other types of cancer.
| Business Type | Exporter, Supplier |
| Form | Powder |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
| Business Type | Exporter, Supplier |
| Form | Tablets |
| Treatment | Cancer |
| Brand Name | Uptravi |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
Selexipag is used to treat high blood pressure in the lungs (pulmonary arterial hypertension-PAH). It is used to help slow down worsening of PAH and to decrease the chance of needing treatment in a hospital. Selexipag works by making it easier for blood to flow through the arteries in your lungs. This effect helps increase your ability to exercise.
Selexipag, sold under the brand name Uptravi, is a medication developed by Actelion for the treatment of pulmonary arterial hypertension (PAH). Selexipag and its active metabolite, ACT-333679 (or MRE-269, the free carboxylic acid), are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation. It is taken by mouth or administered intravenously.
| Business Type | Exporter, Supplier |
| API Form | Liquid |
| Usage | Cancer |
| Dosage | As per Doctor's Prescription |
| Storage | Cool and Dry Place |
| Best Before | 36 Months from Manufacturing |
Preferred Buyer From
| Location | Worldwide |
Vinorelbine (NVB), sold under the brand name Navelbine among others, is a chemotherapy medication used to treat a number of types of cancer. This includes breast cancer and non-small cell lung cancer. Vinorelbine was approved for medical use in the United States in 1994. It is on the World Health Organization’s List of Essential Medicines. Vinorelbine is approved for the treatment of non-small-cell lung cancer. It is used off-label for other cancers such as metastatic breast cancer. It is also active in rhabdomyosarcoma.
Vinorelbine is approved to treat metastatic non-small cell lung cancer (NSCLC). Lung cancer is the second most commonly diagnosedTrusted Source type of cancer worldwide (11.4 percent), after female breast cancer (11.7 percent). NSCLC is also the most common type of lung cancer.
NAVELBINE is a vinca alkaloid indicated:
In combination with cisplatin for first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC)
As a single agent for first-line treatment of patients with metastatic NSCLC
DOSAGE AND ADMINISTRATION
- In combination with cisplatin: 25 to 30 mg/m2 as an intravenous injection or infusion once weekly
- Single agent: 30 mg/m2 as intravenously once a week
- Adjust dose in patients with decreased neutrophil counts or elevated serum total bilirubin
DOSAGE FORMS AND STRENGTHS
Injection: 10 mg/1 mL and 50 mg/5 mL in single-dose vial